By Prof. Dr. Monika Cziferszky:
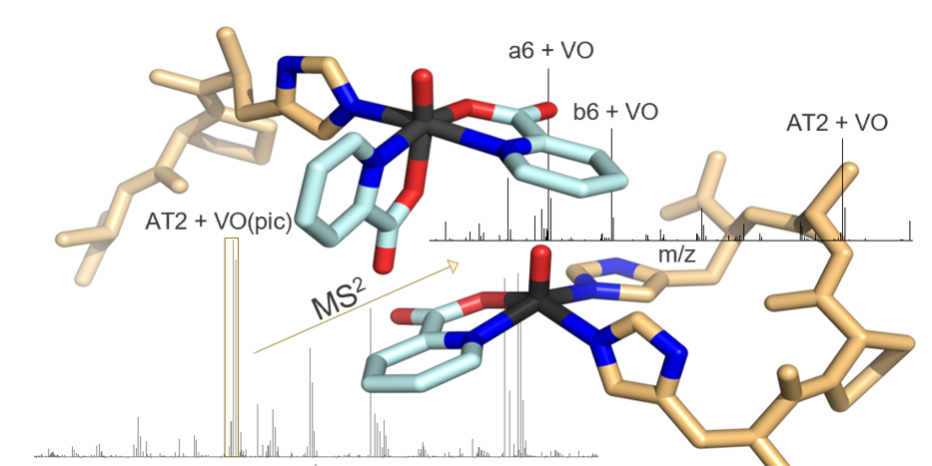
Investigating the speciation of vanadium complexes in the presence of potential biomolecular targets under physiological conditions remains challenging and further experimental techniques are needed to better understand the mechanism of action of potential metallodrugs. A comprehensive analysis of the binding motifs between two model peptides (angiotensin I and angiotensin II) and three well-known oxidovanadium(IV) compounds with antidiabetic and/or anticancer activity, [VO(pic)2(H2O)], [VO(ma)2] and [VO(dhp)2] (where pic−, ma− and dhp− are picolinate, maltolate, and 1,2-dimethyl-3-hydroxy-4(1H)-pyridinonate anions) were obtained by ESI-MS/MS (electrospray ionization tandem mass spectrometry). The results demonstrated that vanadium-peptide bonds are preserved after HCD fragmentation, allowing for the identification of binding sites through detailed analysis of the fragmentation spectra using the Apm2s software tool provided by the open-access EPFL toolbox for mass spectrometry. Insights from this study pave the way to ESI-MS/MS investigations of more complex systems including target proteins and further development of vanadium-based drugs.
Link to the paper: