STEP 3
Peak picking in the chromatogram
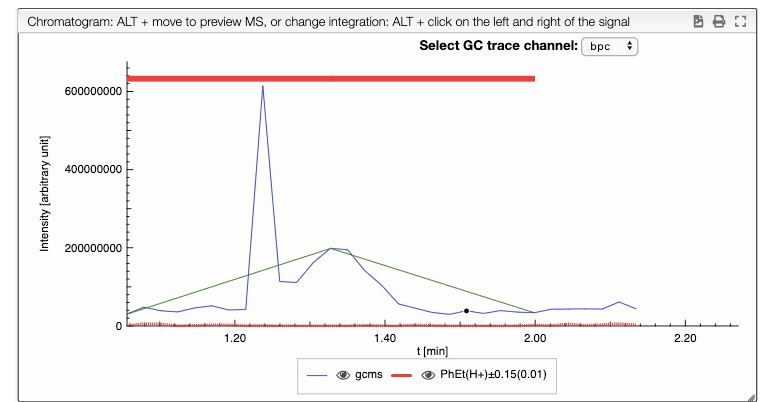
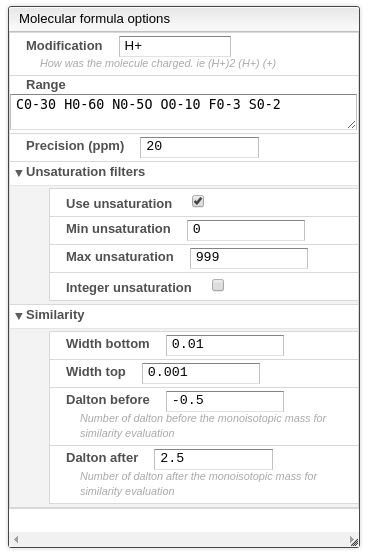
The result of all the selected peaks are stored in the browser, allowing to safely reload the page and continue working from the current selection. Each peak consist of a retention time, the retention time when it begins, the retention time when it ends, the integral between these values, the relative integral and the calculated monoisotopic mass. Each peak also have a zoom action, that allows to easily visualize the peak region. This peaks in the plot also have a red rectangle that indicates their position and a green line that joins the beginning and the ending points of the peak.
Automatic peak picking
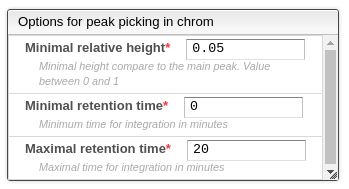
The automatic peak picking task is done using some parameters that allows to filter and improve the results. This peak picking is based on the analysis of the first and second derivative of the chromatogram, therefore the beginning of the peak is where there’s an inflection point. The parameters are the following:
- Minimal relative height: the minimal ratio between the height of the current peak and the highest peak.
- Minimum retention time: the minimal retention time for the integration.
- Maximal retention time: the maximal retention time for integration.
Manual peak picking
All the previous peaks can be modified : First select the corresponding line in the list of peaks, and then you should ALT + Click one at the beginning of the peak and once and the end of the peak in the chromatogram. This is also truth for new peaks. This peaks can be created using the button below the peaks table or clicking on the plus icon over the table.
Note: the automatic peak picking is going to replace the current peak selection, therefore is recommended to use it before the manual peak selection